Introduction
Effective pharmaceutical waste management is crucial for healthcare facilities striving to comply with regulations and protect public health. As environmental sustainability gains importance, the selection and management of non-hazardous pharmaceutical waste containers have emerged as a key concern. Organizations must ensure they are properly segregating and disposing of these materials in line with changing regulations. By examining best practices and guidelines, healthcare providers can navigate this complex landscape, improving operational efficiency and contributing to a safer environment.
Define Non-Hazardous Pharmaceutical Waste
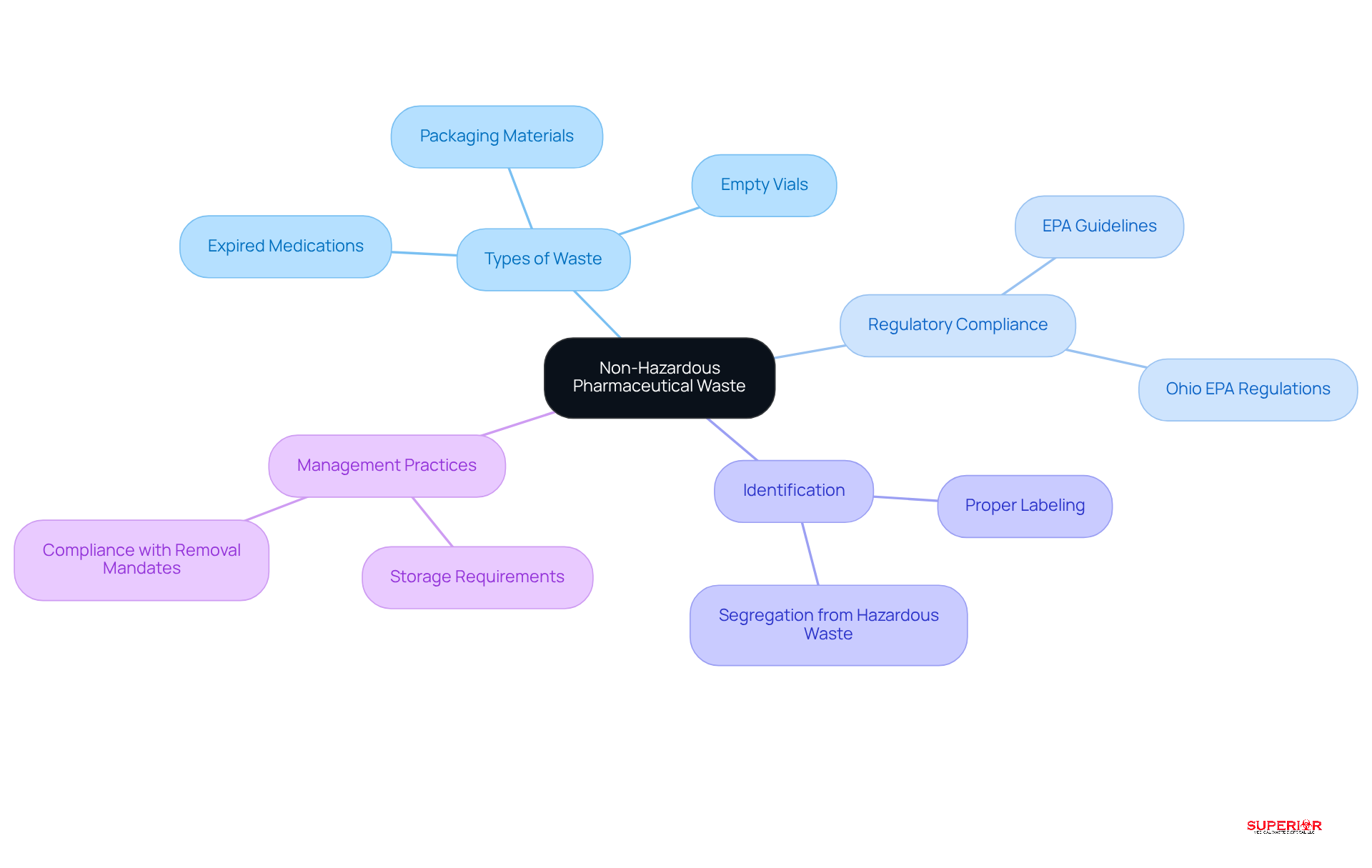
Non-hazardous pharmaceutical refuse encompasses medications and related materials that do not meet the criteria for hazardous substances as defined by the Environmental Protection Agency (EPA). This category includes expired or unused medications, empty vials, and packaging materials free from hazardous substances. Identifying this classification is crucial for healthcare organizations to comply with management regulations, particularly those set forth by the Ohio EPA. For instance, over-the-counter medications and certain prescription drugs that are not classified as hazardous under the Resource Conservation and Recovery Act (RCRA) fall within this category.
Accurate identification of non-hazardous pharmaceutical waste containers is vital for effective segregation from hazardous materials, thereby reducing contamination risks and promoting safe disposal practices. Additionally, all medical refuse-producing facilities in Ohio are legally mandated to have their sharps and biohazard materials removed every 90 days or less, underscoring the importance of compliance in refuse management. Facilities must also adhere to specific storage requirements, ensuring that refuse is kept in a non-putrescent state and stored in non hazardous pharmaceutical waste containers that are properly labeled. By successfully implementing these guidelines, facilities not only enhance their compliance but also contribute to environmental sustainability and public health safety.
Select Appropriate Non-Hazardous Pharmaceutical Waste Containers
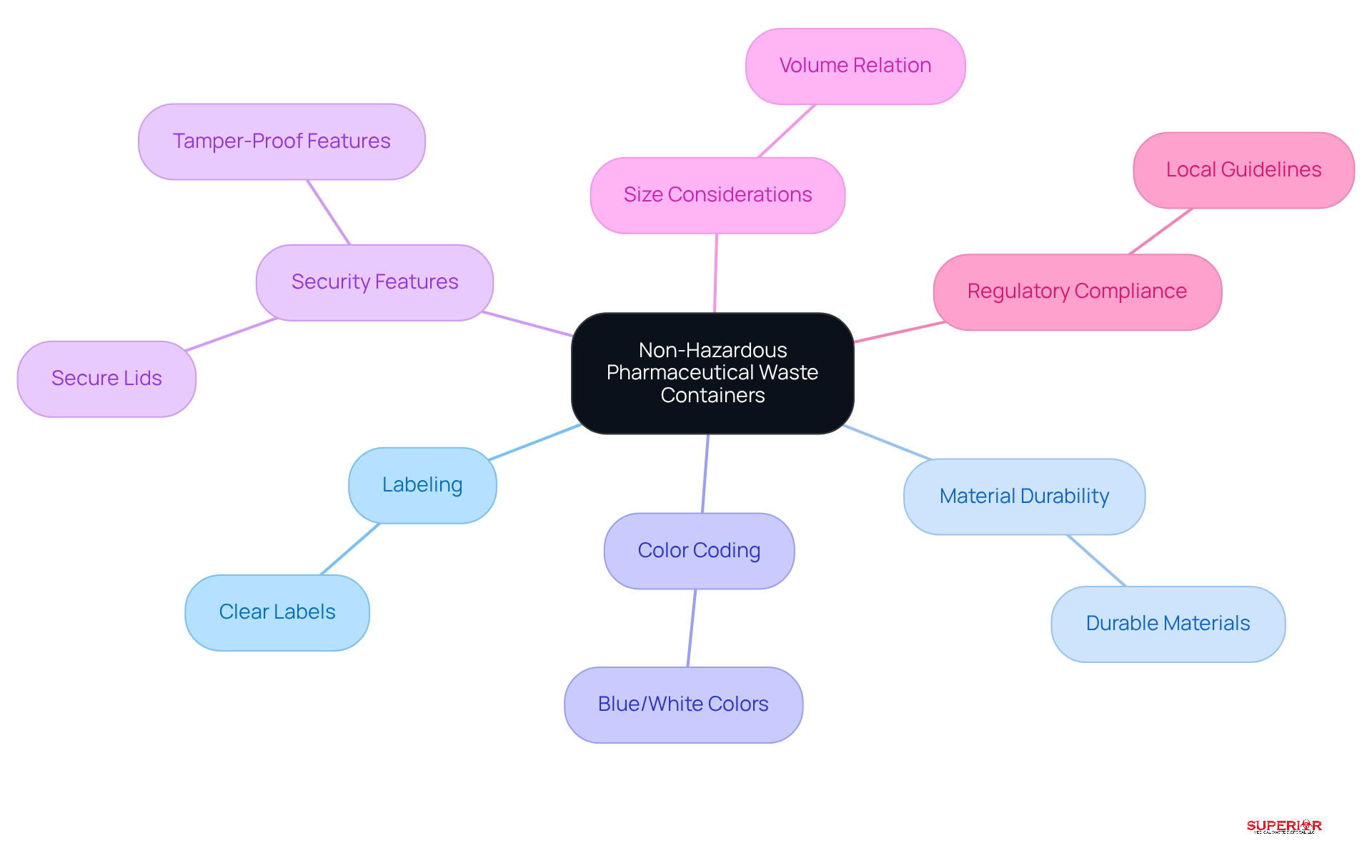
When selecting non hazardous pharmaceutical waste containers for non-hazardous pharmaceutical materials, it is crucial to choose options that are clearly labeled and specifically designed for this type of waste. These non hazardous pharmaceutical waste containers should be made from durable materials capable of withstanding potential leaks and should be easily identifiable, often using blue or white colors to distinguish them from other hazardous waste containers.
Containers equipped with secure lids and tamper-proof features are advisable to prevent unauthorized access and ensure safety. Additionally, facilities must consider the size of the containers in relation to the volume of waste generated and the frequency of disposal. Regular inspections for damage or leaks are essential to maintain safety standards.
It is important to recognize that pharmaceutical waste is classified as universal waste and must be managed separately in accordance with state regulations, such as those in Michigan, Ohio, and Indiana. Healthcare facilities should remain informed about local guidelines to ensure proper disposal practices and compliance with Ohio EPA regulations.
Implement Proper Segregation and Disposal Procedures
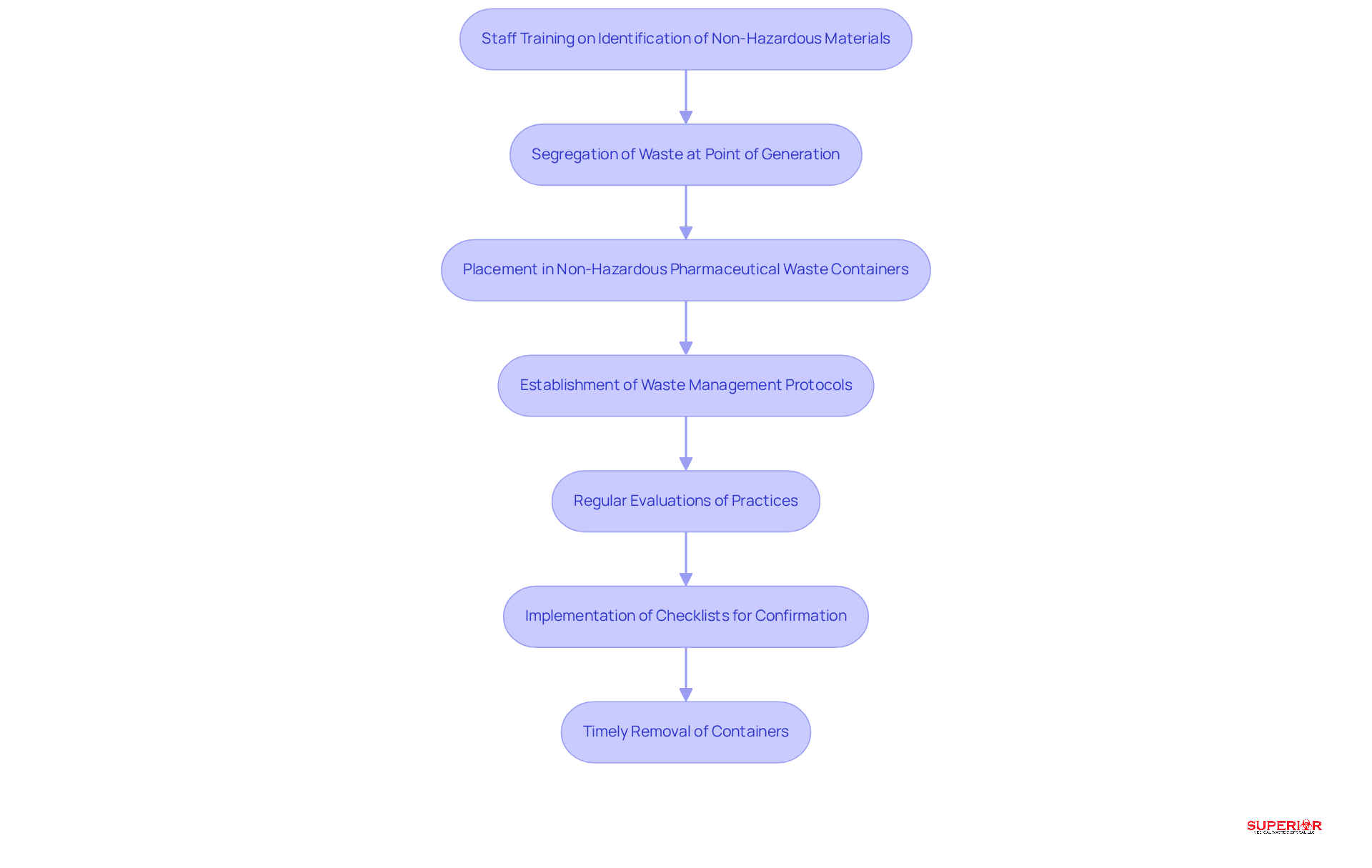
Effective management of non-hazardous pharmaceutical waste containers relies on stringent segregation procedures within healthcare facilities. It is essential for staff to receive thorough training, enabling them to accurately identify non-hazardous materials at the point of generation and promptly place them in non-hazardous pharmaceutical waste containers.
Establishing clear waste management protocols is vital; options may include incineration or compliance with local regulations. Regular evaluations of waste management practices are necessary to identify areas for improvement and ensure adherence to state and federal regulations.
For instance, leading healthcare institutions in Traverse City, such as Munson and Byers Heather, have successfully implemented these practices, demonstrating the effectiveness of proper waste management. Utilizing checklists can help confirm accurate material segregation and facilitate the timely removal of containers, thereby enhancing operational efficiency and regulatory compliance.
Superior Medical Disposal underscores the significance of following established protocols and provides comprehensive solutions for managing pharmaceutical materials in Traverse City and Indiana. By adopting these practices, healthcare organizations can enhance operational efficiency and regulatory compliance, ensuring safe waste management and adherence to all relevant regulations.
Ensure Staff Training and Regulatory Compliance
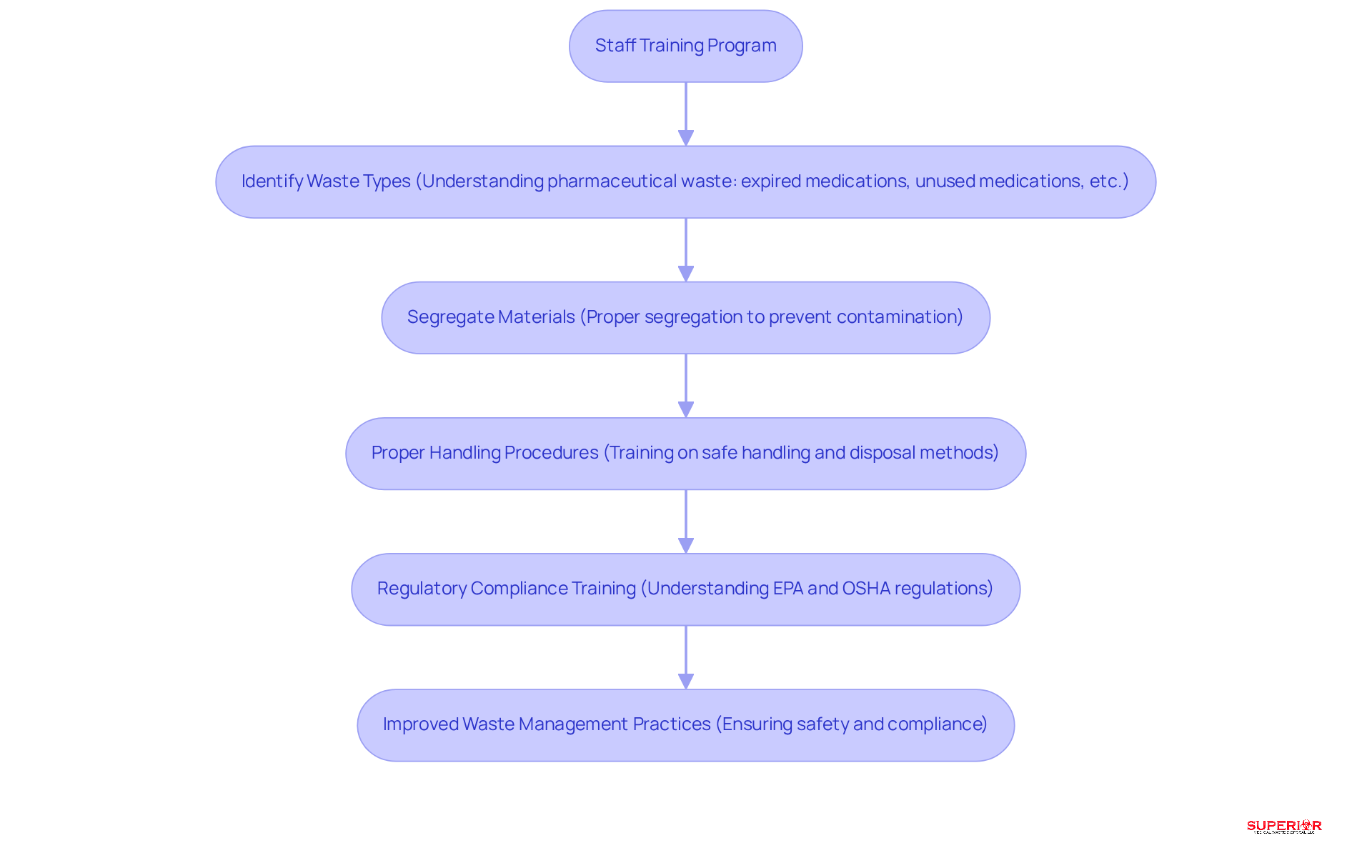
Effective management of non-hazardous pharmaceutical waste containers relies heavily on comprehensive staff training within healthcare facilities. This training must encompass the identification, segregation, and proper handling of these materials, ensuring that all personnel are well-informed about the relevant regulations set forth by the EPA and OSHA. Regular refresher courses and evaluations are crucial, especially considering that only 58% of healthcare professionals express confidence in safely disposing of medical and pharmaceutical materials generated in at-home settings.
Creating a culture of safety and responsibility is vital; healthcare establishments should encourage employees to report any issues or concerns related to waste management. This proactive strategy not only promotes continuous improvement but also aligns with the increasing focus on regulatory compliance training, which is essential for maintaining safe working conditions and avoiding potential penalties.
By investing in robust training programs, including specialized services such as biohazard disposal, document destruction, and the proper use of non-hazardous pharmaceutical waste containers, healthcare facilities can significantly improve adherence to waste management protocols. This investment ultimately ensures a safer environment for both staff and patients.
Conclusion
Effective management of non-hazardous pharmaceutical waste is crucial for healthcare facilities, not only to comply with regulations but also to promote environmental safety. By accurately identifying and utilizing appropriate waste containers, organizations can successfully segregate non-hazardous materials from hazardous waste. This practice minimizes contamination risks and ensures safe disposal.
Key insights emphasize the necessity of:
- Selecting clearly labeled containers made from durable materials
- Implementing stringent segregation procedures
- Providing comprehensive staff training
Facilities must align their waste management practices with local regulations, which include regular inspections and evaluations to enhance compliance and operational efficiency. The experiences of leading healthcare institutions illustrate that adherence to these practices not only improves safety but also cultivates a culture of responsibility among staff.
Given the critical role that proper pharmaceutical waste management plays in safeguarding public health and the environment, it is imperative for healthcare organizations to prioritize these best practices. Investing in staff training and establishing robust waste management protocols will ensure compliance with regulatory requirements and contribute to a sustainable future. By embracing these strategies, healthcare environments will become safer, positively impacting community health.
Frequently Asked Questions
What is non-hazardous pharmaceutical waste?
Non-hazardous pharmaceutical waste includes medications and related materials that do not meet the criteria for hazardous substances as defined by the Environmental Protection Agency (EPA). This category encompasses expired or unused medications, empty vials, and packaging materials that are free from hazardous substances.
Why is identifying non-hazardous pharmaceutical waste important for healthcare organizations?
Identifying non-hazardous pharmaceutical waste is crucial for healthcare organizations to comply with management regulations, particularly those established by the Ohio EPA. Proper identification helps in effective segregation from hazardous materials, reducing contamination risks, and promoting safe disposal practices.
What types of medications are considered non-hazardous?
Non-hazardous medications include over-the-counter medications and certain prescription drugs that are not classified as hazardous under the Resource Conservation and Recovery Act (RCRA).
What are the legal requirements for medical refuse-producing facilities in Ohio regarding waste removal?
Medical refuse-producing facilities in Ohio are legally mandated to have their sharps and biohazard materials removed every 90 days or less.
What are the storage requirements for non-hazardous pharmaceutical waste?
Facilities must adhere to specific storage requirements, ensuring that non-hazardous pharmaceutical waste is kept in a non-putrescent state and stored in properly labeled containers.
How does compliance with non-hazardous pharmaceutical waste guidelines benefit facilities?
By successfully implementing guidelines for non-hazardous pharmaceutical waste management, facilities enhance their compliance, contribute to environmental sustainability, and promote public health safety.
